computerist
school
St. Petersburg
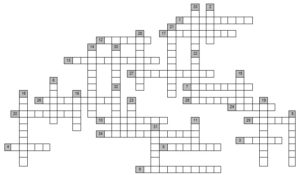
Vertical:
1. The name of the furnace in which cast iron is produced
2. Solvent, additive to motor fuels; formula - C6H6
3. Ammonium chloride. Reacting with other bases, releases ammonia
4. Painful state of the body in case of poisoning with toxic substances
5. Compound formed in substitution reactions of a-amino acids
8. Value measured in square metres
9. Alloy 54-90% copper and 46-10% zinc
10. Substance capable of glowing in the dark
11. Compound in which the formulas of two subsequent compounds differ by CH2
12. Positively charged ion
14. White powder. Supports combustion; formula: NaNO3
17. General name of substances that release energy during combustion
18. Name of the lattice of matter consisting of atoms
21. Colorless gas, odorless, non-combustible, lighter than air; formula N2
22. Proton charge sign
24. The process that occurs with the proton during neutralization, hydrolysis
25. Element of the seventh main subgroup
29. Electron charge sign
30. chemical vessel
32. Silver nitrate
33. Compound serving for the production of dyes; formula - C6H5OH
35. Hydrocarbon; formula - CnH2n
36. Styrene polymerization product, used for the manufacture of toys
38. Salt obtained by decomposition of carbonic acid
39. Thick transparent liquid produced by chemical processing of fats
45. An atomic group whose carbon atom has an unpaired electron
46. Group of one carbon atom and three hydrogen atoms
47. Colorless gas, odorless, flammable, explosive; formula CH4
48. Hydrocarbon, paraffin; formula - CnH2n+2
49. A radical produced from an alkane molecule
50. Component of starch, the molecules of which are bound in the form of a spiral
51. The scientist who discovered the law of electrolysis
54. Mineral raw materials for the production of aluminum
58. Positively charged part of an atom
59. Transparent colorless liquid, hydrogen and oxygen compound
60. Drugs and painkillers extracted from poppy juice
61. A scientist named after a constant in the formula N=Nl*n
62. Substance used for the synthesis of ammonia, in autogenic welding
63. Salt formed during hydrochloric acid dissociation
64. Formaldehyde, has a disinfectant effect; formula - HCHO
65. The form of matter of which all bodies are composed
66. Name of the acid used in oil refining; formula - H2SO4
67. Device for cleaning liquids, gases
68. Colorless gas, supports combustion; formula - O2
69. Substance with a molar mass of 9 g/mol
74. Salt formed during dissociation of phosphoric acid
75. Unit of energy measurement in the SI system
Horizontal:
3. General name of aqueous solutions that do not conduct electric current
6. ethylene polymerization product; used as packaging material
7. The effect of the catalyst on the chemical reaction of biochemical processes
13. The scientist who determined the number of molecules in one mole of matter
15. The name of a number showing the sum of protons and neutrons in an atom
16. Liquid saturated with non-soluble droplets of another liquid
19. Unit of energy equal to 4.19 J
20. Alloy 70-96% copper and 30-4% tin
22. A positively charged particle in the nucleus of an atom
23. Protein containing protein and non-protein parts in macromolecule
26. Substance used to coat metals against corrosion
27. Soft, gray scaly mass, conductor of heat, electricity
28. General name of the substance used in chemical processes
31. Salt formed by decomposition of sulfuric acid
34. Raw materials for glass production; silicon dioxide
36. Potassium carbonate; white powder, easily soluble in water
37. Substance used for alcohol products; formula - C2H5OH
40. Tool in the form of forceps for taking a chemical substance
41. High-molecular substance obtained synthetically
42. The scientist who defined the acid by dissociating it in an aqueous solution
43. Substance with the number of protons in the nucleus equal to 103
44. Colorless, chlorine-containing liquid used for anesthesia
47. fission vessel
49. Messaging energy particles to loosen bonds during impact
52. Salt formed from acetic acid
53. Poisonous substance used as an anesthetic, drug
55. Connection and interrelationship of some phenomena
56. Substance giving red colour to burner flame
57. Acid, respiratory poison; formula - HCl
59. The scientist who created a generator for water gas
60. Substance with good malleability, heat and electrical conductivity
62. Raw materials for reactions with oxygen and nitrogen; refrigerant
70. Substance used for the manufacture of mirror surfaces, contacts
71. Substance, shiny base metal, ferromagnetic, oxidized
72. Organic compound used for the production of dyes
73. High molecular weight compound consisting of homogeneous repeating groups of atoms
76. Salt reaction with water
77. The process of decay of organic substances caused by microorganisms
78. Calcium sulfate; raw materials for the production of sulfuric acid
79. Acid produced by glucose oxidation
80. The process of isolation of pure metal on the electrode during a chemical reaction
81. A complex carbohydrate whose molecule consists of two disaccharides
{module Yandex}
Answers:
Vertical:
1. Domain
2. Benzene
3. Ammonia
4. Toxicosis
5. Dipeptide
8. Square
9. brass
10. phosphorus
11. Homologian
12. cation
14. Nitrate
17. Fuel.
18. Atomic
21. nitrogen
22. Plus.
24. Transfer
25. halogen
29. Minus
30. flask
32. Lapis
33. phenol
35. Cycloalcan
36. Polystyrene
38. carbonate
39. glycerol
45. Radical
46. methyl
47. Methane
48. alkan
49. alkyl
50. Amylose.
51. faraday
54. bioxit
58. Core
59. Water.
60. morphine
61. loschmidt
62. Hydrogen
63. chloride
64. methanal
65. Substance
66. Sulfur
67. Filter
68. Oxygen
69. beryllium
74. phosphate
75. Joel
{module Igraza_mini}
Horizontal:
3. non-electrolyte
6. Polyethylene
7. Biocatalysis
13. avogadro
15. Massive
16. Emulsion.
19. caloria
20. bronze
22. proton
23. Protein
26. nickel
27. Graphite
28. Chemical
31. sulfate
34. quartz
36. potash
37. ethanol
40. Tweezers
41. Plastics
42. Arrhenius
43. Lawrence
44. chloroform
47. Menzurka
49. Activation
52. acetate
53. Cocaine
55. Law
56. lithium
57. Salt
59. Winkler
60. Metal
62. Air.
70. Silver
71. Iron
72. Anilin
73. polymer
76. Hydrolysis
77. Fermentation
78. anhydritis
79. Glucon
80. Electrolysis
81. oligosaccharide